The analysis presented here follows up on earlier fitting of regression models on statistics (like S) based on read counts; somewhat less relevant the subsequent comparison of regression models. Data processing steps as well as several notations and computational objects were introduced in the article on data import.
library(ggplot2)
library(lattice)
library(latticeExtra)
## Loading required package: RColorBrewer
##
## Attaching package: 'latticeExtra'
## The following object is masked from 'package:ggplot2':
##
## layer
Changes in filtering
The original filtering setup
Previous analysis set up two filters in order to reduce error by removing “noisy” or “weak” units of data; the principal difference between the two are the kind of data units they operate on:
- observations i (RNA samples/individuals)
- genes g
Given gene g filtering observations was done according to the total read count associated with each observation Nig, using the rule that i is filtered out if Nig≤50. (Note that more generally g may index a data set aggregated over a set of genes using for instance weighted or unweighted average aggregation method denoted as WA and UA, respectively).
Filtering for genes was then preformed using the rule that a gene be removed if the number of observations that passed the observational filter is <180: “We examined genes where we had greater than 180 analyzable individuals[…]” (see manuscript).
What’s new
Weighting instead of filtering observations
Filtering units in general can be considered as “all or none” weighting so that weight wi of unit i is 1 if i passed the filter and 0 otherwise. It is theoretically beneficial to replace this crude weighting scheme with a more subtle one that uses finer grained weights because that way relatively strong observations that would be filtered out are still allowed to provide some information and, on the other hand, relatively week observations that would pass the filter have smaller impact on the results.
In the current application, given i,g, the total read count lends itself as a natural weight, so wig=Nig. Such weighting scheme is natural if we assume that the higher read count Hig=SigNig is binomial with denominator Nig, which is the case for logistic regression logi.S. Under the normal linear regression model using Sg, Qg and Rg as response there is no such “natural” argument but it is still tempting and seemingly reasonable to use Nig as weight. In the computational objects below this will be denoted as wnlm.S, wnlm.Q, and wnlm.R, whereas no weighting will be labeled with unlm.S, unlm.Q, and unlm.R, respectively (see fit-glms.R).
Weighting genes instead of filtering them
The original rule for genes has little advantage if one recalls that fitting regression models provides standard errors and p-values for regression coefficients so that genes with sparse data will tend to be associated with large errors and insignificant results. As we will see, all but one of the candidate imprinted genes would have to be removed under the <180 rule.
The only advantage of filtering out those genes was to prevent them from inflating the variance of Si (or Qi, Ri) in aggregated data sets that were obtained by unweighted averaging of Sig (or Qi, Ri) over a gene set G. The new implementation of data import and processing (see import-data.R) introduces weighted average as a method of aggregating Sig for a set of genes. Again, this is natural if Sig,g∈G are assumed binomial with corresponding denominators Nig and, in addition, assumed to share pi≡pig1=pig2=.... The new implementation also calculates the unweighted average UA, but the present analyses will be based only on WA. This way, genes will affect the results from aggregated data sets in proportion with their contribution to those data.
Extended data set
Load functions…
source("~/projects/monoallelic-brain/src/import-data.R")
source("~/projects/monoallelic-brain/src/fit-glms.R")
Obsolete: Select the following (candidate) imprinted genes:
# 8 genes analyzed by Ifat
gene.ids <- c("PEG3", "INPP5F", "SNRPN", "PWAR6", "ZDBF2", "MEG3", "ZNF331", "GRB10",
# 5 more genes analyzed by AGK 3/2/16
"PEG10", "SNHG14", "NAP1L5", "KCNQ1OT1", "MEST",
# 3 more genes present in data files
"IGF2", "NLRP2", "UBE3A",
# 'green' novel 1 MB imprinted genes; note that PWAR6 is already included above
"TMEM261P1", "AL132709.5", "RP11-909M7.3", "SNORD116-20", "RP13-487P22.1", "hsa-mir-335", "PWRN1")
Update following readjustment of calling monoallelic genes (in a later post) select these genes:
gene.ids <- unlist(read.csv("../../data/genes.regression.new", as.is = TRUE))
names(gene.ids) <- gene.ids
Get data: observations on predictors (explanatory variables) and on the higher and lower read count from selected genes (more details in a previous post)
E <- get.predictors() # default arguments
Y <- get.readcounts(gene.ids = gene.ids, count.thrs = 0)
Y.f <- get.readcounts(gene.ids = gene.ids, count.thrs = 50)
The number of available observations
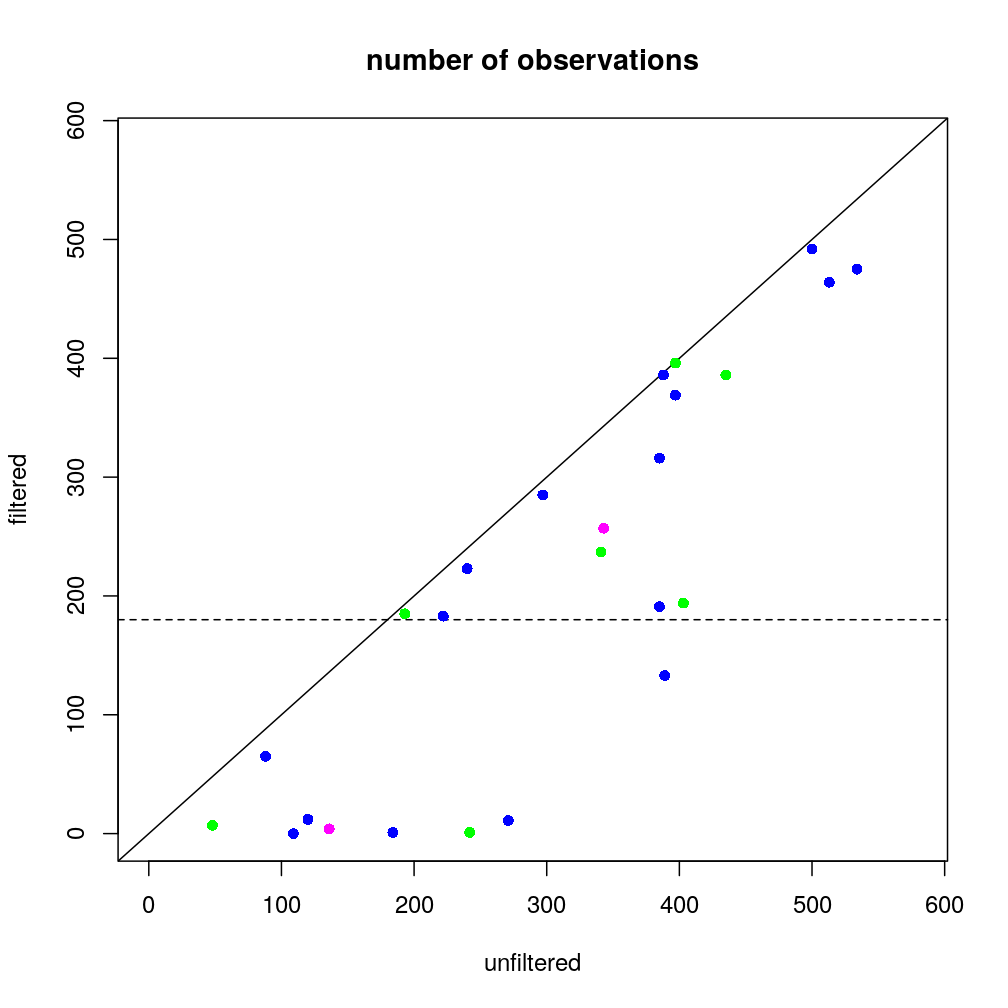
The number of available observations is closely (and inversely) related to the standard error of estimated regression coefficients such as of ˆβage, on which our attention is centered. The number of observations before and after filtering at total read count threshold ≤50)…
(nobs <- as.data.frame(lapply(list(unfiltered=Y, filtered=Y.f), function(y) sapply(y, function(x) sum(! is.na(x[[1]]))))))
## unfiltered filtered
## MAGEL2 184 1
## TMEM261P1 109 0
## SNHG14 534 475
## AL132709.5 389 133
## RP11-909M7.3 271 11
## ZIM2 240 223
## NAP1L5 222 183
## MEG3 513 464
## PEG3 500 492
## PWAR6 388 386
## FAM50B 120 12
## NDN 88 65
## SNURF 297 285
## PEG10 397 369
## SNRPN 385 316
## KCNQ1OT1 385 191
## ZDBF2 435 386
## GRB10 403 194
## SNORD116-20 193 185
## KCNK9 242 1
## INPP5F 397 396
## RP13-487P22.1 48 7
## MEST 341 237
## ZNF331 343 257
## hsa-mir-335 136 4
## DIRAS3 44 0
## PWRN1 155 10
## IGF2 183 14
## NLRP2 177 28
## UBE3A 94 19
## WA.8 577 566
## WA 579 579
## UA.8 577 560
## UA 579 578
which shows that the largest trade-off of filtering arises for genes for which the number of observations is ab ovo small, in particular for most genes in the “novel 1 MB” category. The same information is plotted below; blue denotes known imprinted genes, green candidate imprinted genes (<1 MB) and magenta two gene sets aggregated by weighted average (WA, overlapping each other). The horizontal dashed line shows the filtering threshold of 180 observations that was defined previously to remove genes with little data.
Conclusion
The above results show that the previously used gene filter would remove 6 out of the 7 candidate genes with which the analysis now is extended. This points to one of the benefits of the new weighting schemes introduced above.
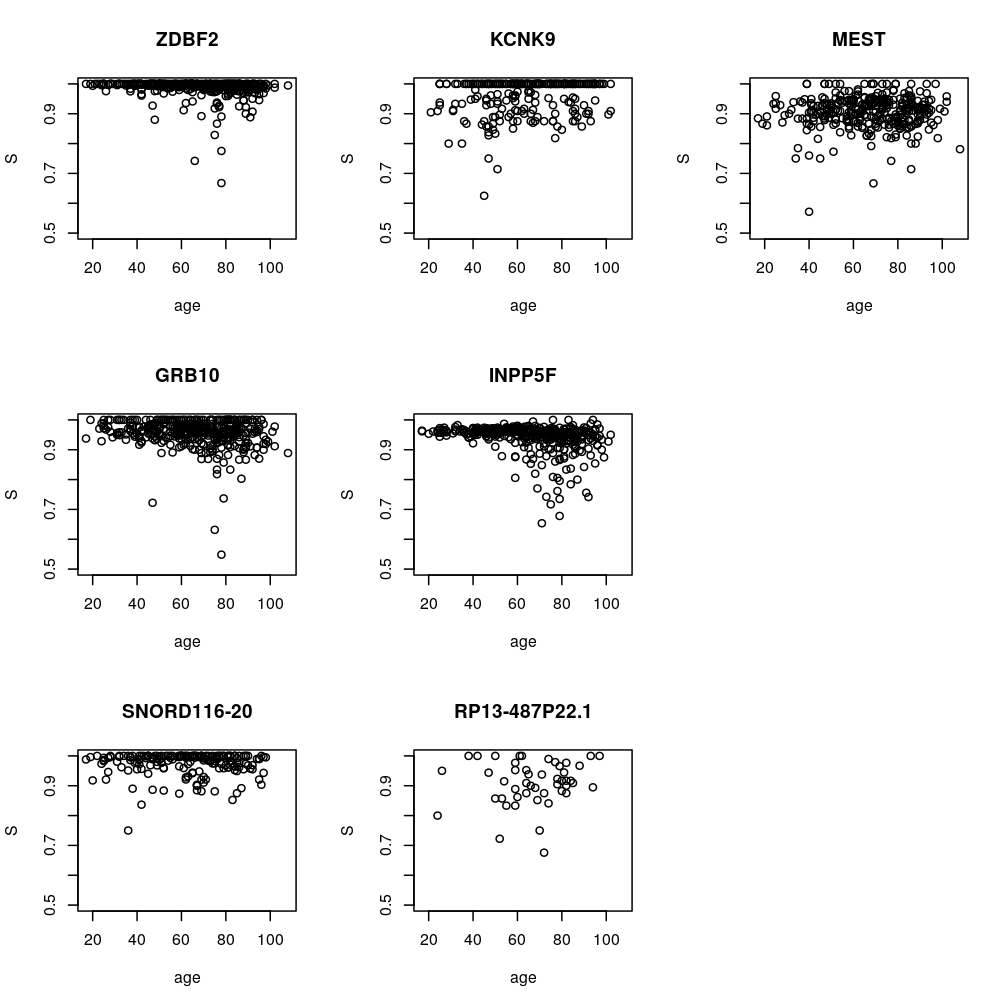
Apparent age dependence of Sg for candidate genes g
Estimation of regression coefficients
Fitting models
Fitting all models to all retained gene-wise and aggregated read count data sets
# exclude unweighed aggregates UA.8 and UA from fitting
to.fit.ids <- grep("^UA(.8)?$", names(Y), value = TRUE, invert = TRUE)
to.fit.ids <- grep("^WA.8$", to.fit.ids, value = TRUE, invert = TRUE)
M <- do.all.fits(Y[to.fit.ids], preds = e.vars)
## Warning: glm.fit: algorithm did not converge
## Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
## Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
## Warning: glm.fit: algorithm did not converge
## Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
## Warning: glm.fit: fitted probabilities numerically 0 or 1 occurred
The following models have been fit…
names(M)
## [1] "unlm.Q" "wnlm.Q" "unlm.R" "wnlm.R" "unlm.S" "wnlm.S" "logi.S"
## [8] "logi2.S"
…to the following data sets
names(M[[1]])
## [1] "MAGEL2" "TMEM261P1" "SNHG14" "AL132709.5"
## [5] "RP11-909M7.3" "ZIM2" "NAP1L5" "MEG3"
## [9] "PEG3" "PWAR6" "FAM50B" "NDN"
## [13] "SNURF" "PEG10" "SNRPN" "KCNQ1OT1"
## [17] "ZDBF2" "GRB10" "SNORD116-20" "KCNK9"
## [21] "INPP5F" "RP13-487P22.1" "MEST" "ZNF331"
## [25] "hsa-mir-335" "DIRAS3" "PWRN1" "IGF2"
## [29] "NLRP2" "UBE3A" "WA"
The warnings arising during the fit also reflect the fact that for TMEM261P1 the fit failed to converge both under logi.S and logi2.S, so let
f.ids <- as.data.frame(lapply(M, function(m) ! sapply(m, is.null)))
f.ids["TMEM261P1", c("logi.S", "logi2.S")] <- FALSE
Note that potential NULL results reflect genes that have been filtered out—by default none.
Regression coefficient βage
Logistic model
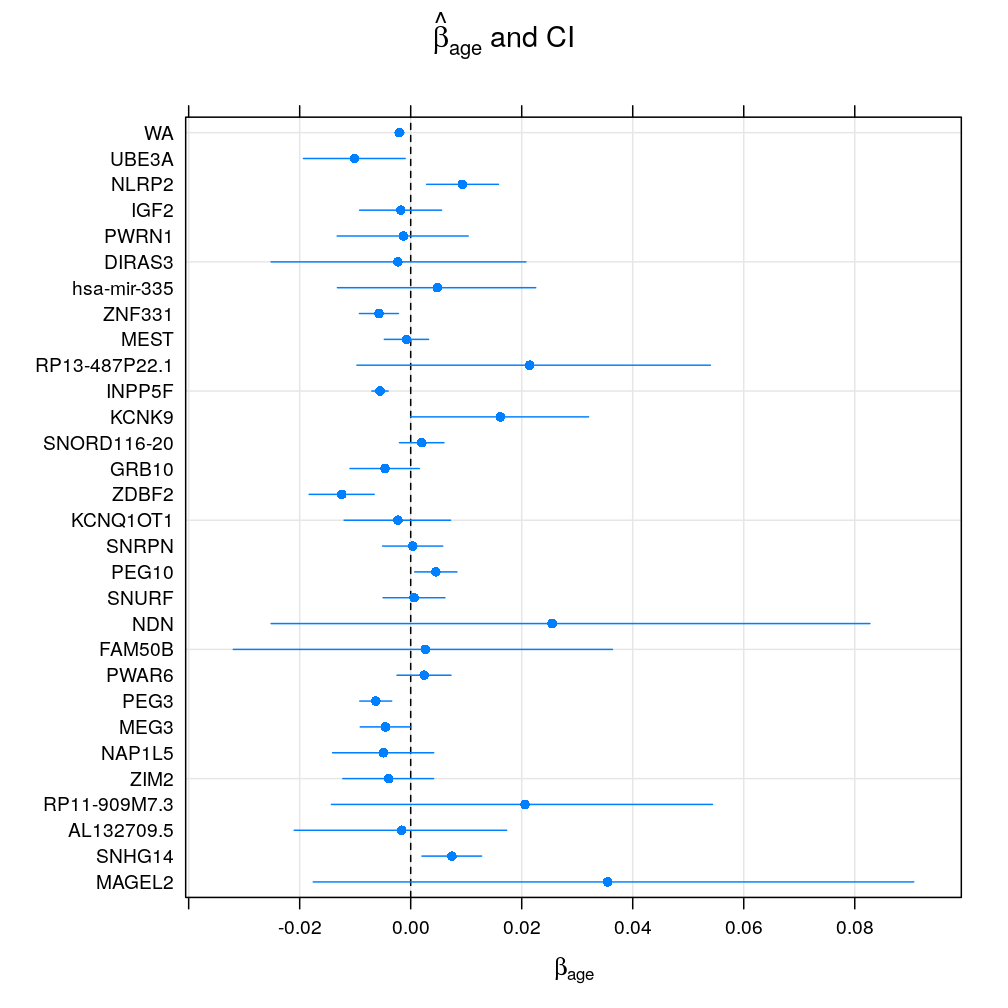
Under the logi.S model βage seems to vary greatly accross genes suggesting both gain or loss of imprinting with age or no effect of age. Genes with fewer observations (as typical for candidate imprinted genes) have broad confidence intervals and so tend support the null hypothesis of βage=0 at a given significance level α. Aggregating read counts using weighted average is hugely beneficial as evidenced by dramatically shrunken confidence intervals (WA) because the logistic model takes advantage of the increased total read counts. The following pair of plots (obtained with the lattice and ggplot2 package, respectively) illustrate these result:

Normal linear model on quasi-log-transformed data
Under the wnlm.Q model the above picture slightly changes. In general, confidence intervals are broader than under logi.S. In particular, the normal linear model fails to benefit from data aggregation by ignoring total read counts; therefore the confidence intervals for WA are not shrunken relative to single-gene data sets under this model. The lattice and ggplot2 implementation, as above:
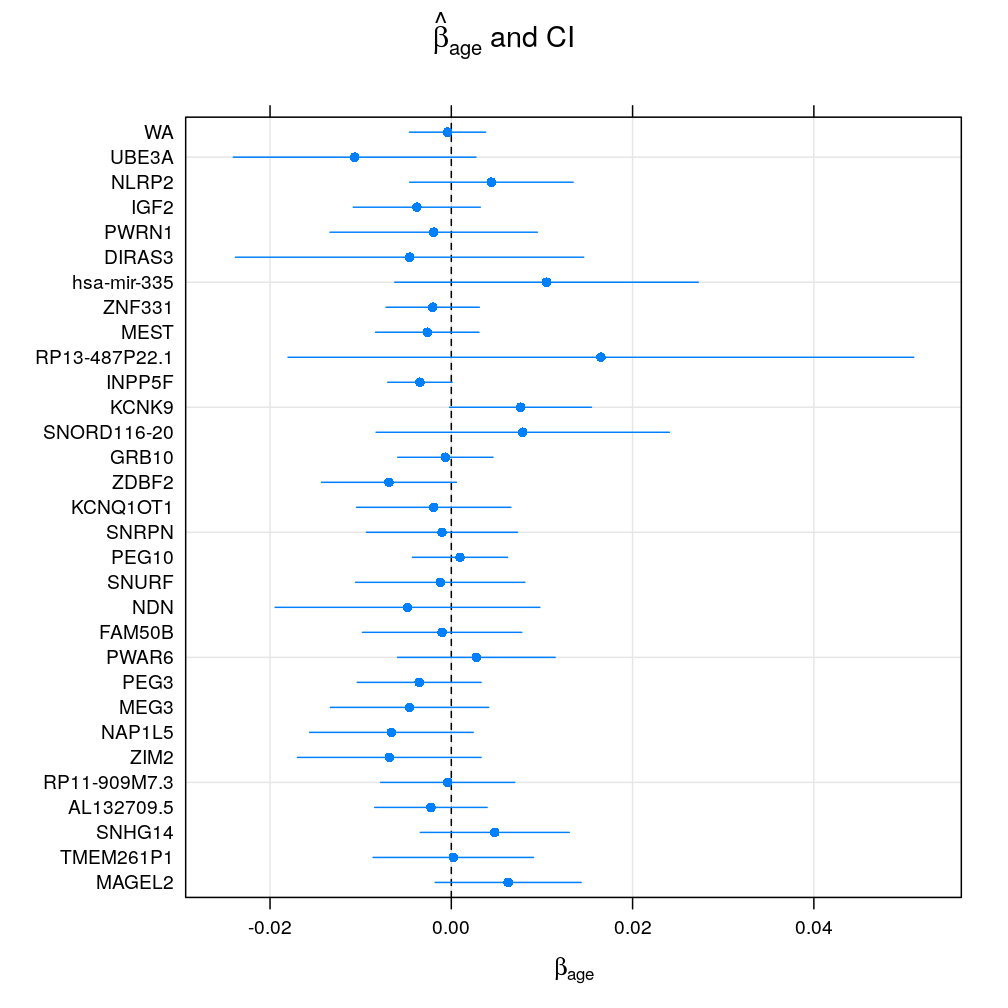

Comparing βage from different models
Consistency between wnlm.Q and logi.S
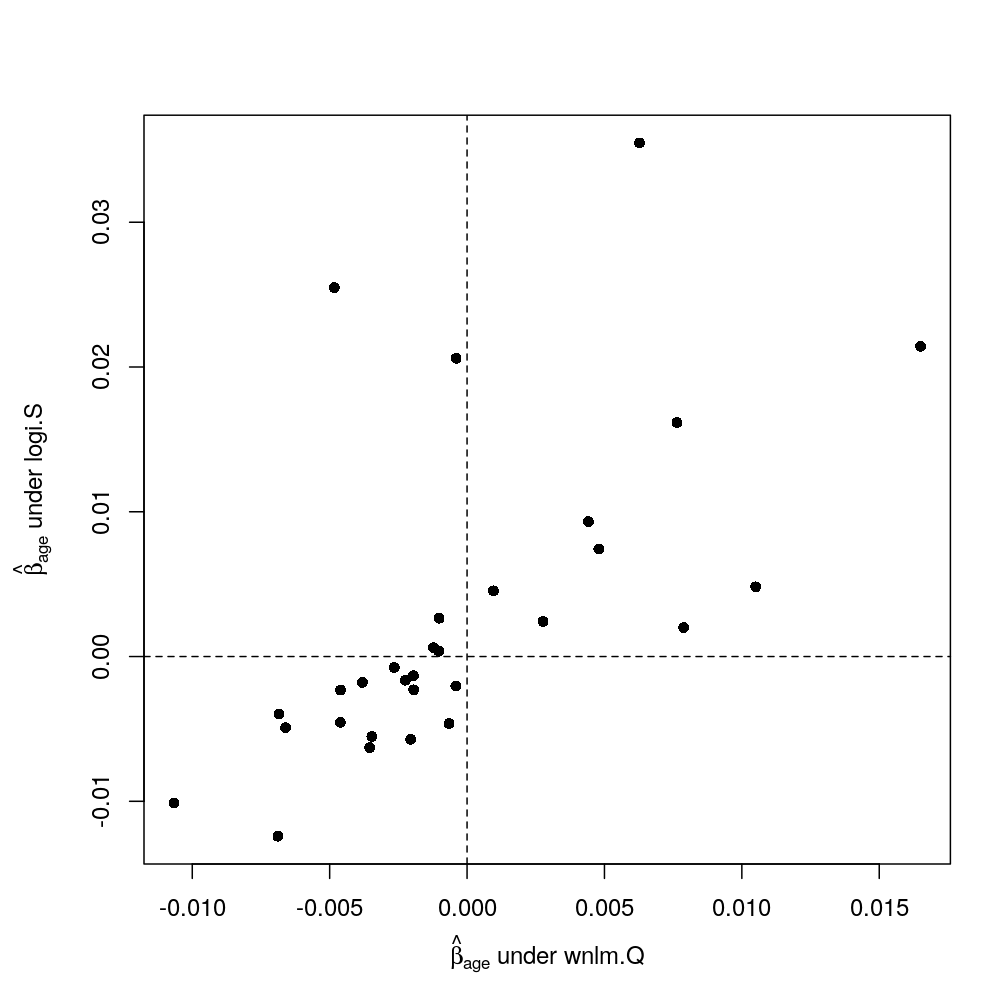
Impact of S rescaling (logi2.S) and of weighting observations (nlm)
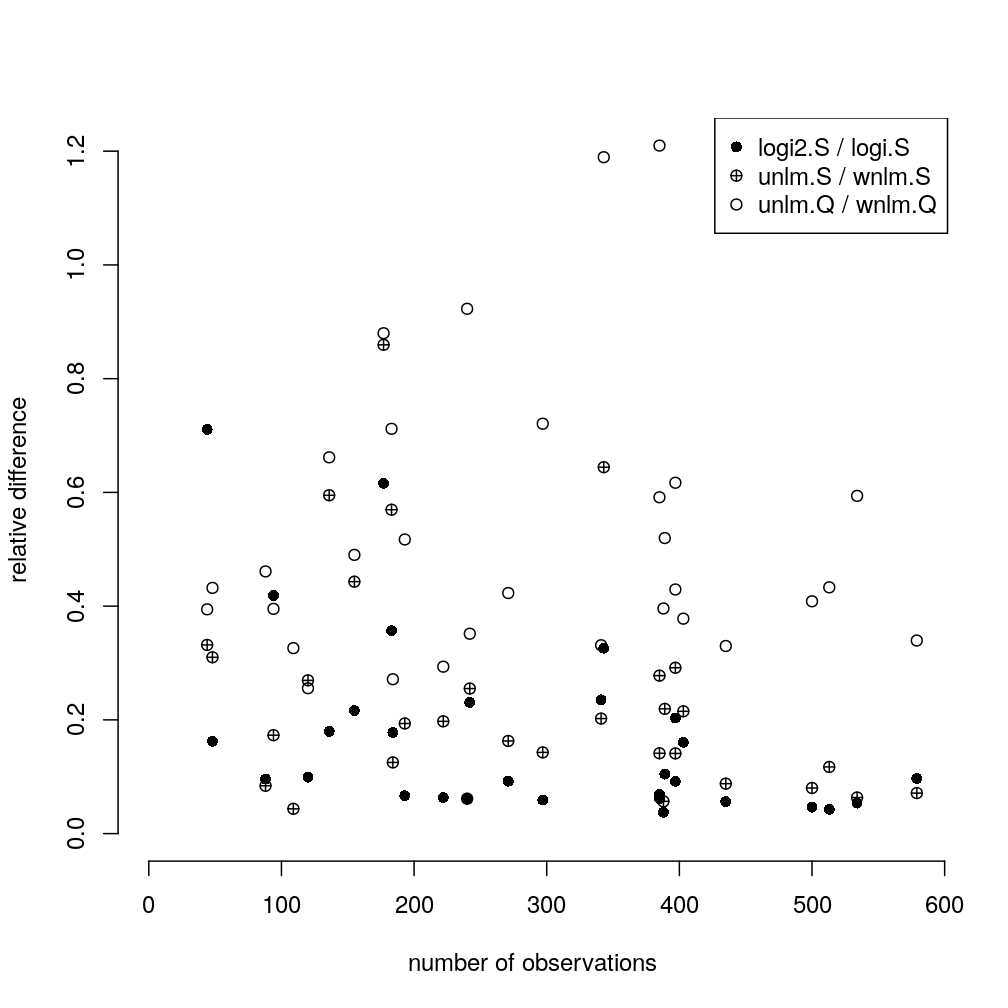
Let M′/M denote the comparison of two models in terms of the mean absolute difference of regression coefficients under M′ relative to M for a given gene or aggregate g, defined as ∑j|β′jg−βjg|∑j|βjg| where {βjg∣j=1,..,p} and {β′jg∣j=1,..,p} is a set of p regression coefficients under M and M′, respectively.
The following plot shows such differences for three model comparisons and all genes and aggregates. Two systematic trends be seen:
- the difference tends to decrease with increasing number of observations indicating that some of the difference comes from sampling error due to limited number of observations
- the
logi2.S/logi.Sdifferences (both can be considered weighted averages) tend to be smaller than the differences between weighed and unweighted averaging of {Sg}g and especially that of {Qg}g.